Helping advance our industry’s understanding of the challenges and opportunities we face
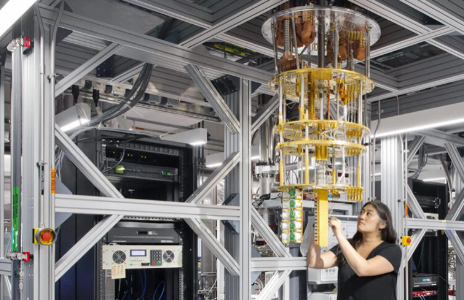

Medical Design & Outsourcing published a byline by Axel Wirth and Naomi Schwartz exploring how post-quantum cryptography (PQC) will reshape medical device security. The article urges manufacturers to act now to future-proof their products against emerging quantum threats and highlights how proactive preparation aligns with FDA cybersecurity expectations.
























.png)
.png)
.png)
.png)














































No News Found
Show moreWe are dedicated to helping advance our industry’s understanding of the challenges and opportunities we face through research. Check out our whitepapers.
Watch our latest webinar that discusses regulatory updates and the impact for medical device development and post-market management.

No matter where you are in the regulatory submission process, we have a variety of services that can meet your needs when and where you need us.


The Guardian platform is a secure and scalable cryptographic solution that simplifies security processes and incident response.


Gain visibility across your software supply chain to detect, prioritize, and remediate cybersecurity risk.
